by adminmps | Sep 6, 2022 | News, Resources
Want to find out more about anaesthesia and how it relates to rare diseases? OrphanAnesthesia publishes anaesthesia recommendations for rare diseases in order to facilitate the work of anaesthesiologists and to improve patients’ safety. This a project of the...

by adminmps | Sep 6, 2022 | News
The Department of Health and Human Services (DHHS) in the United States has approved adding MPS II as a condition to the recommended uniform screening panel (RUSP) for newborns. This enables several states to begin testing newborns immediately. Early diagnosis...

by adminmps | Sep 6, 2022 | Clinical Trials, News, Research
In our March edition we reported that Abeona Therapeutics had decided to end enrollment for their clinical trial for MPS IIIA children. We are now happy to share that Ultragenyx will be taking over the clinical trials for AAV gene therapy ABO-102 (now UX111). “Based...

by adminmps | Sep 6, 2022 | Clinical Trials, News, Research
We are excited to share the news that REGENXBIO Inc. has announced its intention to file a Biologics License Application (BLA) in 2024 using the FDA’s accelerated approval pathway for the treatment of Hunter Syndrome, Mucopolysaccharidosis Type II (MPS II). The...

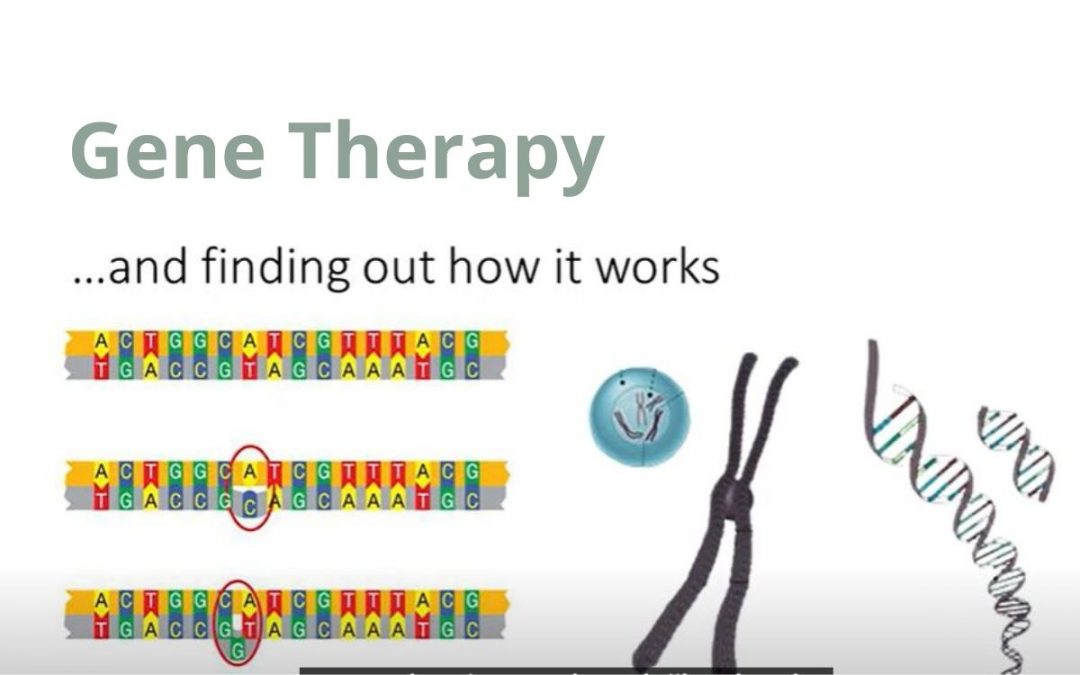
by adminmps | Sep 6, 2022 | News, Resources
Ever wondered… How does gene therapy work?Why does it work better for some conditions that others?What is the difference between in vivo and ex vivo gene therapy?What are the ways of receiving gene therapy?How safe is it and what are the dilemmas surrounding gene...